2017–First Pipeline for Proteomics-based Clinical Diagnostics — published on PNAS
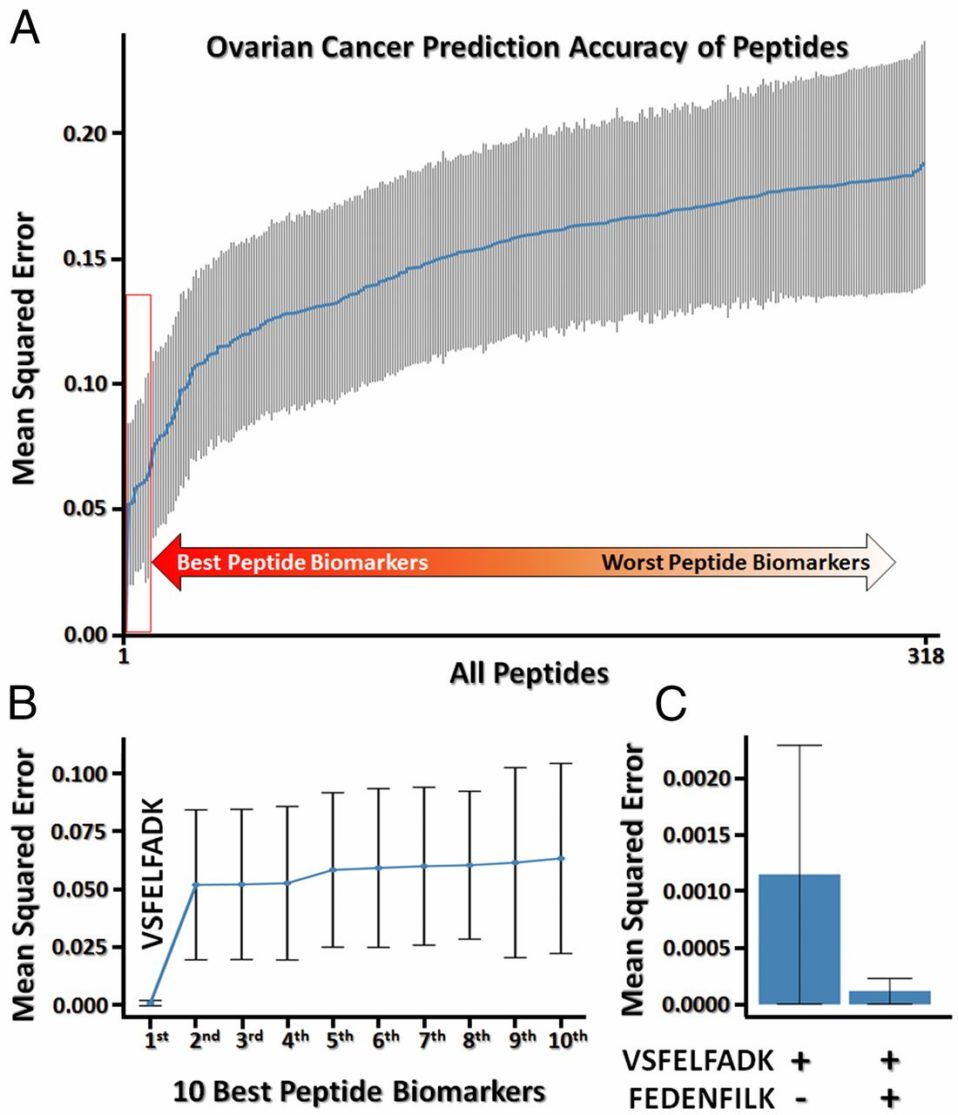
We developed the 1st pipeline for discovering and validating proteomic biomarkers from patient plasma samples (Ref 1). With this pipeline, we identified an ovarian cancer biomarker, peptidyl-prolyl cis–trans isomerase A (PPIA), with zero false positive diagnostic value. Our finding was featured on Genomeweb (Ref 2).
We quantified 318 peptides in 94 separate samples, 48 from healthy controls, 14 from colorectal cancer cases, 14 from ovarian cancer cases, and 18 from pancreatic cancer cases. We then analyzed this data to see if any of the peptides or combinations of the peptides allowed us to accurately identify their sample of origin, and in this way identified the two PPIA peptides as potential markers for ovarian cancer. We then measured these two peptides in a separate cohort of 73 cases consisting of 35 ovarian cancer cases and 14 healthy controls and 24 pancreatic cancer cases. One of the two peptides was detected in 20 of the 35 ovarian cancer plasma samples, while neither was detected in any of the healthy controls.